PERIPHERAL INTERVENTION
Our goal at Marcus Medical, and as well as our principle suppliers, is to serve as a procedural partner to physicians by providing the tools needed to effectively access, target, and treat this complex vascular disease, preserving lives and limbs.
Access Technology
Access devices are the products used to gain and maintain percutaneous entry into the blood circulation. A small puncture site made through the skin allows interventional tools entry to treat endovascular pathologies through minimal invasive techniques. Various access sites can be selected in the body to gain entry into either the arterial or venous system. The most frequently selected sites are the common femoral, radial, brachial, axillary, popliteal and tibiopedal arteries. For veins the most common access sites are femoral, jugular and popliteal veins.
Accessories
Olcott Torque Device is used to facilitate directional control of wire guides. Unique single-handed operation permits rapid position changes during procedural use. Single size allows use with standard and hydrophilic wire guides ranging in size.

Catheters
Beacon® Tip radiopacity ensures excellent visibility during catheter manipulation. Hydrophilic coatings provide long-lasting lubricity to smooth navigation—even through tortuous anatomy. Braided stainless steel construction offers 1:1 torque response, as well as superior pushability and kink-resistance. Gradual transition of radiopaque Beacon tip to catheter shaft results in stability during injection. Nylon material resists softening during prolonged catheter manipulation. The CXI® Support Catheters has stainless steel braiding that provides superior pushability. Four radiopaque markers allow vessel segment sizing. The distal 40cm portion has hydrophilic coating for ease of insertion and passage through tortuous anatomy. Depth markers clearly show progression into the sheath. Low profile and Cook’s superior tip tapering process facilitate passage through tight lesions providing limb preservation solutions in the treatment of peripheral arterial and venous disease.

Introducers
Introducers are our most diverse product ranges in peripheral intervention consisting of comprehensive ranges of access and guiding sheaths; sizes available from 4Fr to 18Fr. Flexor® Technology is available where access is difficult or where delivery is demanding. Introducers with Flexor technology have been developed to address everything from the everyday to the most challenging access. They are kink resistant and has patented coil-reinforced construction that provides optimal flexibility and maximum resistance to compression. The radiopaque band seamlessly incorporated within the sheath material, identifies precise location of the distal tip for positioning accuracy and enhanced visability. Selected configurations are available with AQ®hydrophilic coating to dramatically reduce friction and enhance trackability. Large lumens with low-friction inner surface facilitate unconstrained device delivery .
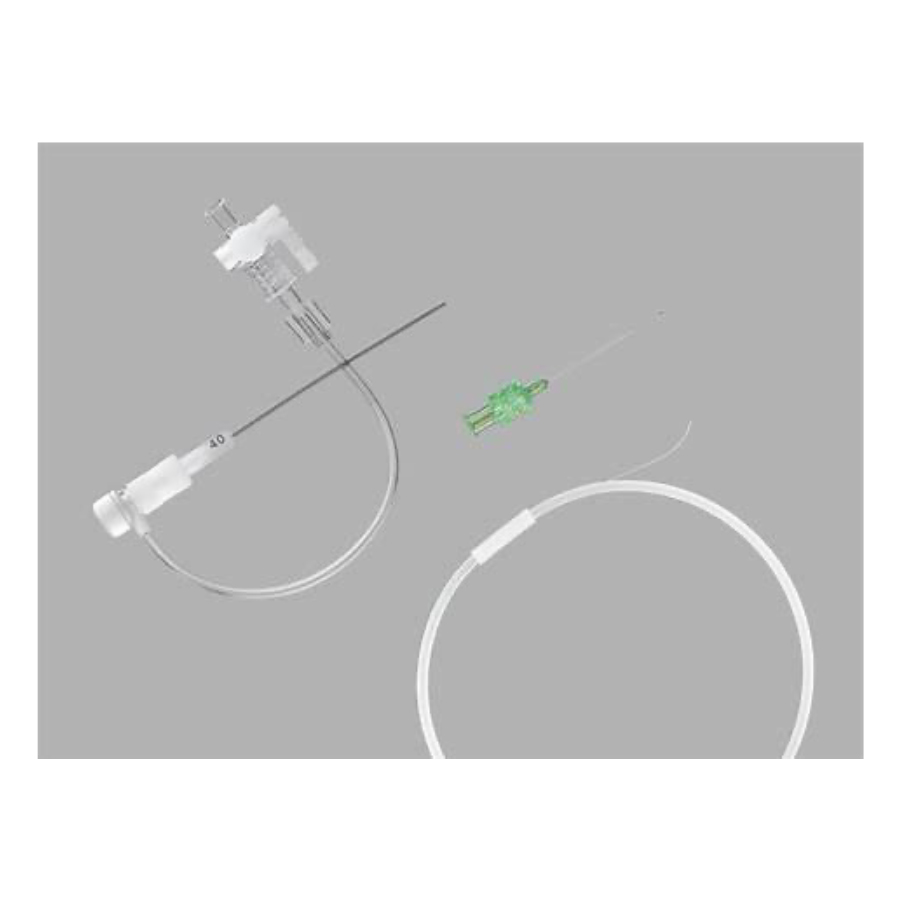
Micropuncture
Micropuncture access sites result in fewer complications. In a study by Shah et al, access site bleeding complication rates dropped from 11.7% to 2.6% when Micropuncture was used. The coaxial catheter pairs provide smooth entry with the Transitionless-Tip™ that has been engineered to glide into the vessel with less force. The sharp 21 gauge needle features EchoTip® technology that enhances ultrasound visibility. The supportive nitinol wire has a palladium tip for excellent visibility under fluoroscopy.


Needles
Access begins with a Vascular Seldinger Disposable needle. These needles will facilitate the placment of a 0.035/0.038″ diameter wire guide through the inner lumen of the puncturing device.


Wire Guides
Wire guides are used to enter obstructed vessels, within the body, or to assist in inserting, positioning, and moving a catheter. Wire guides vary in size, length, stiffness, composition, and shape of the tip. Five decades of partnerships between Cook Medical and visionary interventionalists have culminated in one of the world’s broadest and most advanced offerings of wire guides—each crafted to the highest quality standards.

Peripheral Arterial Disease(PAD) Therapies
Peripheral arterial disease (PAD) occurs when fatty deposits form in arteries that are outside of your heart and can restrict the flow of blood. PAD often occurs in the legs, and in severe cases can lead to amputation. People who have PAD are at greater risk of suffering a heart attack or stroke. PAD is most common in older people, as well as in smokers as smoking can increase the risk of PAD. Other risk factors include Diabetes, Obesity, High blood pressure, Family history of hardening of the arteries (atherosclerosis), High cholesterol, Age, gender and race. Most people with PAD don’t show any warning signs, and symptoms are often mistaken for signs of aging. Symptoms include: leg pain when walking, numbness or weakness in the legs, aching pain in the feet or toes while at rest, ulcers or sores in the leg or foot that won't heal, a cold leg or foot and skin colour changes in the legs or feet. If you have PAD, your doctor may ask you to exercise more and stop smoking if you are a smoker. Your doctor may also prescribe drugs to thin your blood and to lower your cholesterol and blood pressure. All of these actions can help to slow the progression of PAD and also decrease your chances of having a heart attack or stroke. However, drugs and lifestyle changes alone don’t resolve all cases of PAD. For these patients, angioplasty, atherectomy, stent placement, or bypass surgery may be necessary.
Angioplasty
Small balloon catheters are used to open up fatty deposits in your arteries. A broad offering in profile selection is available. A flexible tip enhances your ability to navigate tortuous vasculature. Enhanced Rewrap technology helps maintain sheath compatibility after deflation.


Balloon Expanding Stents
Formula stents do not shorten, and the ends align with the balloon markers. These features help you to place the stent and to cover the lesion reliably and precisely. Precise comformibility is maintained with the additional crowns that improve scaffolding. Formula 418 Renal Balloon-Expandable Stents are indicated for use in patients with atherosclerotic disease of the renal arteries following suboptimal percutaneous transluminal renal angioplasty (PTRA) of a de novo or restenotic lesion (≤ 18mm in length) located within 10mm of the renal ostium and with a reference vessel diameter of 4.0-7.0mm. Suboptimal PTRA is defined as ≥ 50% residual stenosis, ≥ 20mm Hg systolic or ≥ 10mm Hg mean translesional pressure gradient or flow-limiting dissection.


Drug Eluting Stent
Zilver PTX, is the world’s first DES for the treatment of PAD in the superficial femoral artery (SFA). Zilver PTX is coated with paclitaxel. Zilver PTX is a stent that is coated with a drug that helps keep arteries from closing. The stent is used to treat the fatty deposits that can restrict blood flow in the largest artery of the thigh. This vessel is called the superficial femoral artery (SFA). The Zilver PTX stent is made of an alloy (a combination of metals) called nitinol. Things that are made of nitinol return to their original shape after they are squeezed or bent. Nitinol makes the Zilver PTX stent open up by itself when your doctor puts it in your artery. Stents like Zilver PTX that open up by themselves are called self-expanding stents. Zilver PTX is the first stent with a drug coating that the FDA approved for use outside of the heart. Zilver PTX holds open the SFA and delivers the paclitaxel. The drug helps stop tissue growth that could close an artery. This treatment is highly valued by patients because it improves their quality of life and decreases their need for repeat procedures.


Self-Expanding Stents
Stents are used in vessels that do not respond to angioplasty treatment. A stent is a small, metal tube that lines the inside of a fatty deposit and keeps the artery propped open. Zilver Flex® 35 Vascular Stents have Proven Patency: The Zilver® vascular stent has been clinically proven to maintain 90% patency in the iliacs at two years. Proven Clinical Results: Patients experienced significant and sustained improvement in ABI and walking scores at two years. Proven Design: Flexibility and radial force are balanced to achieve excellent conformability and fracture resistance. Indication: Zilver Flex is indicated for Iliac, SFA and above the knee popliteal .

Venous Therapies
Deep vein thrombosis (DVT or a blood clot in the deep veins of the leg) is a common disease state. If the thrombus breaks off (embolizes) and flows towards the lungs, it can become a pulmonary embolism (PE) which is a blood clot in the lungs. Post‐thrombotic syndrome refers to symptoms and signs of chronic venous insufficiency that develops following deep vein thrombosis and is a common, burdensome, and costly complication. These conditions can be debilitating and treatment will improve the quality of life in these patients significantly.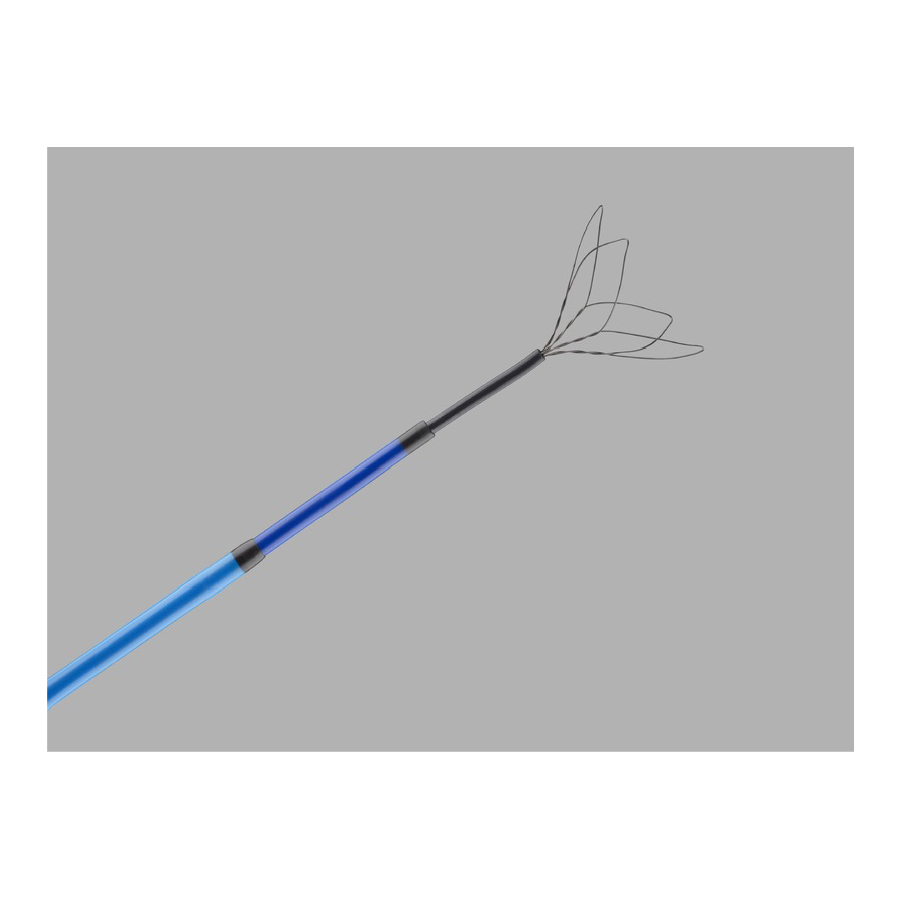
Clover Retrieval Set
CloverSnare® 4-Loop Vascular Retriever. Used in the cardiovascular system to manipulate and retrieve foreign objects, including, but not limited to, wire guides, coils, balloons, catheters, and filters.


Filters
Celect™ Platinum Vena Cava Filter. Used for the prevention of recurrent pulmonary embolism (PE) via placement in the vena cava in the following situations:
- Pulmonary thromboembolism when anticoagulant therapy is contraindicated
- Failure of anticoagulant therapy in thromboembolic diseases
- Emergency treatment following massive PE where anticipated benefits of conventional therapy are reduced
- Chronic, recurrent PE where anticoagulant therapy has failed or is contraindicated. The Cook Celect Platinum Filter implant may be retrieved. The four radiopaque platinum markers can verify placement accuracy immediately after you detach the filter.


Self- Expanding Stents
Zilver® Vena™ – Venous Self-Expanding Stent for Iliofemoral venous obstruction restores venous flow while maintaining flexibility and kink resistance with proven Zilver technology.


Triforce
TriForce® Peripheral Crossing Set Intended to be percutaneously introduced into blood vessels and support a wire guide while performing percutaneous peripheral interventions. This device is also intended for injection of radiopaque contrast media for the purpose of angiography.

Embolic Therapies
With more than 300 different coils Cook Medical offers the broadest range of embolization products on the market . Embolization products include: pushable and detachable coils, PVA particles, microcatheters, and microwire guides. Most coils have a general indication and can be used for both arterial and venous embolization.
Embolisation Coils
Coils come in a variety of shapes: Spiral, Tornado (Helical), Straight, Single Loop and J-shape.
Sizes include .011, .018, .035, and .038 inches covering the need for both micro- and macro embolization. The majority of coils used are made of either platinum or Inconel. The soft texture of platinum enables the coils to conform to the vessel creating a dense cross sectional occlusion. Inconel is a nickel-chromium based alloy. Its composition is firmer than platinum causing these coils to maintain their shape and exceed greater radial force making our MReye Inconel coils ideal for high-flow environments. Most coils are provided with nylon fibres ranging from a few fibres to heavily fibred, depending on the coil type. Fibres increase the thrombogenicity of the coils thus creating faster occlusion


Microcatheter
Cantata® Microcatheter used in small vessel or super selective anatomy for diagnostic and interventional procedures, including peripheral and coronary use. Cook Medical offers both braided and non-braided microcatheters with good control in a variety of sizes and flow rates. The large inner diameter can accommodate embolic particles, spheres or microcoils including all .018 inch embolization coils. Braided construction for superior control, trackability and kink resistance. Lipiodol/DMSO compatibility for a broad range of applications. A hydrophilic coating facilitates introduction and reduces trauma .

Drug Coated Balloons

APERTO OTW
APERTO OTW is a high-pressure paclitaxel releasing haemodialysis shunt balloon dilatation catheter specifically designed for AVF stenosis. APERTO OTW was developed specifically to solve the unmet clinical needs in treatment of haemodialysis access stenosis and recanalization of arteriovenous fistula and central vein obstruction.
APERTO OTW achieves superior primary patency compared to conventional HPB angioplasty in AVF.


LEGFLOW
Paclitaxel Releasing Peripheral Balloon Dilatation Catheters.
With Safepax Cardionovum is leading the way in matrix technology. Safepax is a third-generation paclitaxel balloon matrix coating, developed exclusively for the Cardionovum family of DCB’s for maximal safety and optimised drug delivery.